Ƙungiyar Cyano tana da ƙarfi mai ƙarfi da kuma shaƙar electron, don haka tana iya shiga cikin furotin da aka yi niyya don samar da haɗin hydrogen tare da mahimman ragowar amino acid a wurin aiki. A lokaci guda, ƙungiyar cyano ita ce jikin isosteric na bioelectronic na carbonyl, halogen da sauran ƙungiyoyin aiki, wanda zai iya haɓaka hulɗa tsakanin ƙananan ƙwayoyin magunguna da furotin da aka yi niyya, don haka ana amfani da shi sosai a cikin gyaran tsarin magani da magungunan kashe ƙwari [1]. Wakiliyar cyano da ke ɗauke da magungunan likitanci sun haɗa da saxagliptin (Hoto na 1), verapamil, febuxostat, da sauransu; Magungunan noma sun haɗa da bromofenitrile, fipronil, fipronil da sauransu. Bugu da ƙari, mahadi cyano kuma suna da mahimmancin amfani a fannoni na ƙamshi, kayan aiki da sauransu. Misali, Citronitrile sabon ƙamshi ne na nitrile na duniya, kuma 4-bromo-2,6-difluorobenzonitrile muhimmin abu ne na kayan abinci don shirya kayan lu'ulu'u na ruwa. Ana iya ganin cewa ana amfani da mahadi cyano sosai a fannoni daban-daban saboda keɓancewarsu ta musamman [2].
Ƙungiyar Cyano tana da ƙarfi mai ƙarfi da kuma shaƙar electron, don haka tana iya shiga cikin furotin da aka yi niyya don samar da haɗin hydrogen tare da mahimman ragowar amino acid a wurin aiki. A lokaci guda, ƙungiyar cyano ita ce jikin isosteric na bioelectronic na carbonyl, halogen da sauran ƙungiyoyin aiki, wanda zai iya haɓaka hulɗa tsakanin ƙananan ƙwayoyin magunguna da furotin da aka yi niyya, don haka ana amfani da shi sosai a cikin gyaran tsarin magani da magungunan kashe ƙwari [1]. Wakiliyar cyano da ke ɗauke da magungunan likitanci sun haɗa da saxagliptin (Hoto na 1), verapamil, febuxostat, da sauransu; Magungunan noma sun haɗa da bromofenitrile, fipronil, fipronil da sauransu. Bugu da ƙari, mahadi cyano kuma suna da mahimmancin amfani a fannoni na ƙamshi, kayan aiki da sauransu. Misali, Citronitrile sabon ƙamshi ne na nitrile na duniya, kuma 4-bromo-2,6-difluorobenzonitrile muhimmin abu ne na kayan abinci don shirya kayan lu'ulu'u na ruwa. Ana iya ganin cewa ana amfani da mahadi cyano sosai a fannoni daban-daban saboda keɓancewarsu ta musamman [2].
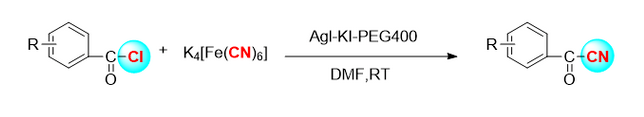
2.2 amsawar cyanidation na electrophilic na enol boride
Tawagar Kensuke Kiyokawa [4] ta yi amfani da sinadaran cyanide n-cyano-n-phenyl-p-toluenesulfonamide (NCTS) da p-toluenesulfonyl cyanide (tscn) don cimma ingantaccen cyanidation na mahaɗan enol boron (Hoto na 3). Ta hanyar wannan sabon tsari, β-Acetonitrile daban-daban, kuma yana da nau'ikan substrates iri-iri.
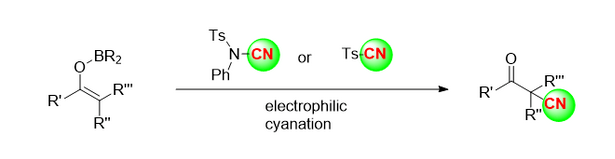
2.3 silico cyanide mai narkewa a cikin ketones
Kwanan nan, ƙungiyar jerin Benjamin [5] ta ba da rahoto a cikin mujallar Nature game da bambancin enantiomeric na 2-butanone (Hoto na 4a) da kuma amsawar cyanide mara daidaituwa na 2-butanone tare da enzymes, masu haɓaka sinadarai na halitta da masu haɓaka ƙarfe na canzawa, ta amfani da HCN ko tmscn azaman mai haɓaka sinadarai na cyanide (Hoto na 4b). Tare da tmscn a matsayin mai haɓaka sinadarai na cyanide, 2-butanone da sauran ketones masu yawa an fuskanci halayen silyl cyanide mai ƙarfi na enantioselective a ƙarƙashin yanayin mai haifar da illa (Hoto na 4C).
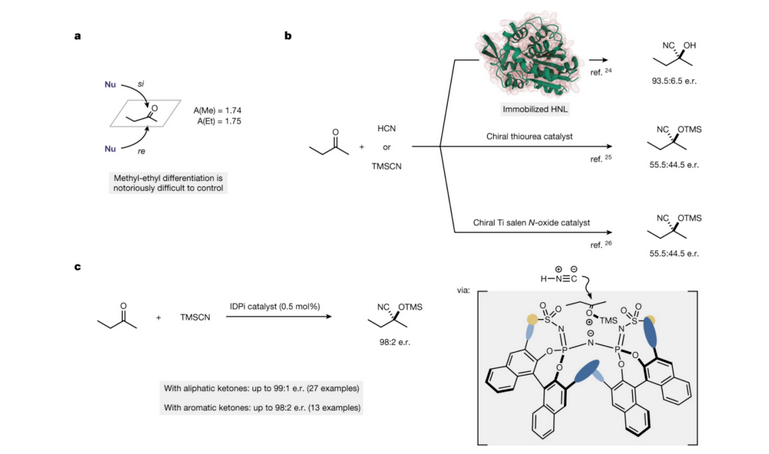
Siffa ta 4 A, bambance-bambancen enantiomeric na 2-butanone. b. Rashin daidaituwa na 2-butanone tare da enzymes, abubuwan haɓaka sinadarai na halitta da abubuwan haɓaka ƙarfe na canzawa.
c. Idpi yana haɓaka amsawar silyl cyanide mai ƙarfi na 2-butanone da sauran ketones iri-iri.
2.4 rage yawan sinadarin aldehydes
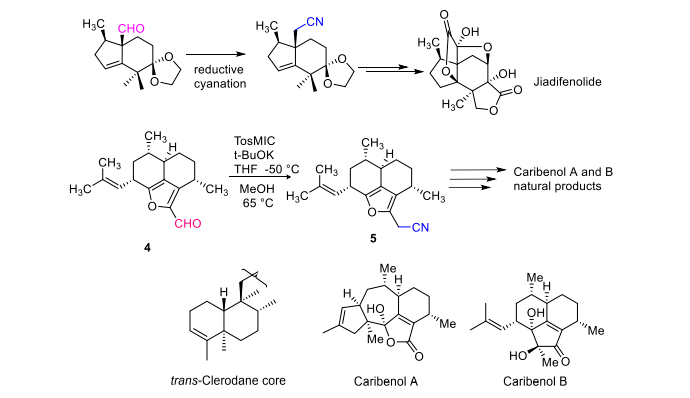
A cikin haɗakar samfuran halitta, ana amfani da tosmic kore a matsayin maganin cyanide don sauƙaƙe canza aldehydes masu hana sterically zuwa nitriles. Ana ƙara amfani da wannan hanyar don shigar da ƙarin atom na carbon cikin aldehydes da ketones. Wannan hanyar tana da mahimmanci mai gina jiki a cikin cikakken haɗin jiadifenolide na Enantiospecific kuma muhimmin mataki ne a cikin haɗakar samfuran halitta, kamar haɗakar samfuran halitta kamar clerodane, caribenol A da caribenol B [6] (Hoto na 5).
2.5 electrochemical cyanide martanin amine na halitta
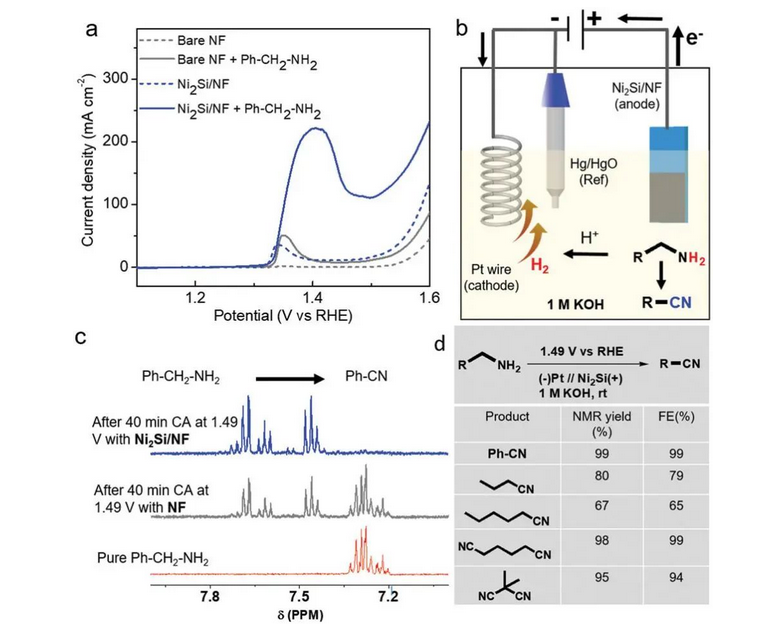
A matsayin fasahar haɗakar kore, an yi amfani da haɗakar sinadarai ta halitta a fannoni daban-daban na haɗakar halittu. A cikin 'yan shekarun nan, masu bincike da yawa sun mai da hankali a kai. PrashanthW. Ƙungiyar Menezes [7] kwanan nan ta ba da rahoton cewa ana iya haɗa amine mai ƙanshi ko aliphatic amine kai tsaye zuwa ga mahaɗan cyano masu dacewa a cikin maganin KOH na 1m (ba tare da ƙara cyanide reagent) tare da yuwuwar 1.49vrhe akai-akai ta amfani da mai rage radadi na Ni2Si, tare da yawan amfanin ƙasa mai yawa (Hoto na 6).
Takaitaccen bayani na 03
Cyanidation wani muhimmin abu ne na haɗakar sinadarai na halitta. Tun daga ra'ayin sinadarai masu kore, ana amfani da sinadaran cyanide masu kyau ga muhalli don maye gurbin magungunan cyanide na gargajiya masu guba da cutarwa, kuma ana amfani da sabbin hanyoyi kamar ba su da sinadarai masu narkewa, marasa sinadarai masu guba da kuma hasken microwave don ƙara faɗaɗa fa'idar bincike, don samar da fa'idodi masu yawa na tattalin arziki, zamantakewa da muhalli a fannin samar da masana'antu [8]. Tare da ci gaba da ci gaban binciken kimiyya, amsawar cyanide zai haɓaka zuwa ga yawan amfanin ƙasa, tattalin arziki da kuma ilmin sunadarai masu kore.
Lokacin Saƙo: Satumba-07-2022





